Class 10 Science
Chapter 4 Carbon and its Compounds
Activity Solutions
Activity 4.1
Brief procedure:
Activity 4.1 asks us to list the materials we use in daily life and classify them by their source.
Answer:
In daily life, we see and utilise many materials. We can classify them in many ways like shape and size, composition, colour etc. Based on the composition of the material, we can classify materials like metals, plant products, soil and clay products and so on. Below is the classification of material as metals, clay products and others.
Things made of metal: glass, plate, compass box, pen, bottle, mobile phone, watch, key-ring, fridge, cooler
Items made of glass/clay: Glass, bowl, pitcher, window, glass door.
Example of others: Book, pen, bottle, glass, tiffin box, cooler, washing machine, bag, cloth, wood door, polythene bag etc
Vegetables we eat are organic compounds.
Explanation:
We use a variety of materials in our daily life. A significant number of objects consist of organic compounds. For example, all plant products like fruits, seeds, paper; all soil products like clay pot; all polythene products like plastic, rubber etc. are organic compounds. This vast presence of organic compound signifies its importance in our life.
Activity 4.2
Brief procedure:
Activity 4.2 asks us to observe the difference in formulae and molecular masses of various alcohol.
Observation:
Name: Chemical formula: Difference; Molecular weight: Difference
Methanol: CH3OH —- 32 —–
Ethanol: C2H5OH -CH2 46 14
Propanol: C3H7OH -CH2 60 14
Butanol: C4H9OH -CH2 74 14
Explanation:
Carbon contains four electrons in its valance shell. It needs 4 extra valence electrons to complete the octet. It combines with various other molecules like oxygen, nitrogen, hydrogen but also reacts with other carbon atoms as well. We call such property as polymerisation tendency. Here, a carbon atom attaches to another carbon atom and elongates the chain. The various compounds formed show similar property and gradual change (increase or decrease) in the property, e.g. Boling point, melting point, solubility, polarity. We call such groups as homologous series.
Methanol, ethanol, propanol, butanol etc. are similar in structure and molecular weight increases by 14amu (Atomic Mass Unit). Here Melting point and boiling point also increase with the number of carbon atoms. They all show the polar property due to the presence of polar -OH(hydroxy group). With an increase in the bulkiness of carbon atom, higher alcohols are less polar.
We use such a tendency to predict the behaviour of the molecule.
Similarly, the property of other functional groups like Amide (CONH2), Carboxylic acid (COOH), Primary, secondary, and tertiary Amines (NH) etc. also depend on the number of carbon atoms.
Inference/conclusion: molecules of a homologous series show similar physical and chemical property.
Activity 4.3
Brief procedure:
Activity 4.3 asks us to burn various organic compounds and observe the flame.
Observation:
Naphthalene and camphor burn with yellow flame while alcohol burn with a blue flame.
Explanation:
Structure of Naphthalene, Ethanol, and Camphor
We use Naphthalene to preserve cloth from microorganisms. It is an unsaturated compound with multiple double bonds. It burns in air and gives yellow smoke.
Alcohol and camphor are saturated hydrocarbon. Alcohol burns with a blue flame which show complete combustion. Camphor burn with a yellow flame.
Colour of the flame depends on the amount of carbon relative to others. The higher proportional carbon atom results in incomplete combustion. Naphthalene has five double bonds so it has more carbon atoms; camphor has one cyclic ring so lesser than naphthalene; while ethanol is completely saturated carbon compound without a cyclic ring. As a result, naphthalene has a sooty yellow flame while ethanol has a blue flame.
Activity 4.4
Brief procedure:
Activity 4.4 asks us to examine the flame colour with changing the position of the knob of the Bunsen burner.
A Bunsen burner.
Observation:
The flame changes its colour from yellow to blue with the change in position of the knob.
Explanation:
Bunsen burner is a simple burner which we use in a laboratory.
It consists of a gas pipe, a metal base, barrel and an adjustable collar.
At the bottom of the flame pipe, there are two small holes for air. A small knob, Collar is present near the hole to adjust the air inflow.
If the knob is completely open flame is blue and of smaller height. While if we close the knob the flame height increase with a yellow flame.
Gas is the common LPG gas that we use in daily life. LPG (liquified petroleum gas) is a carbon compound. It is butane the fourth homologous of the simplest carbon compound, methane. Under the insufficient quantity of air, it burns with a yellow flame.
Activity 4.5
Brief procedure:
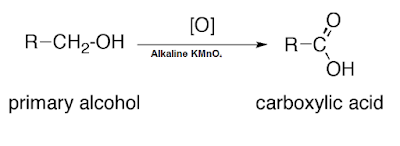
Activity 4.5 asks us to add alkaline potassium permanganate in warm ethanol and observe the change in colour.
Observation:
Initially red colour of potassium permanganate disappear. On adding more potassium permanganate red colour prevail.
Explanation:
Potassium permanganate is a strong oxidizing agent. It oxidizes alcohol to acids and reduces itself to manganese dioxide. Potassium permanganate solution is red in colour. Initially, when we add potassium permanganate all potassium permanganate is used up in the reaction. After completion of the reaction, there is no more ethanol in the solution. Adding more potassium permanganate after this endpoint makes the solution red.
Why we use a water bath?
Alcohol is a highly inflammable substance. Heating it directly may lead to an explosion. A water bath is a beaker filled with water. We add the alcohol in a test tube and place it in a beaker. It warms the solution without catching fire.
Activity 4.6
Brief procedure:
Activity 4.6 asks us to add a few pieces of sodium metal to an ethanol solution and test for the gas evolved.
Observation:
Ethanol reacts with sodium to form sodium ethoxide. This salt is colourless and soluble in water. Hydrogen gas evolves during the process which we check by bringing a flame to the test tube.
2C2H5OH(aq) + 2Na(s) —–> 2C2H5ONa (aq)+ H2(g)
Activity 4.7
Brief procedure:
Activity 4.7 asks us to compare the PH of dilute acetic acid and dilute Hydrochloric acid using litmus paper and universal paper.
Observation:
Acetic acid is a weak acid. Its PH range is 2 to 4. A typical,1M solution of acetic acid has a PH of 2.4. So on litmus paper, it shows a red-yellow colour. See activity 2.11.
Hydrochloric acid is a strong acid. It’s 0.1M solution has a PH of 1. It shows an utterly red colour with litmus paper.
Universal PH indicator:
Universal PH indicator
A universal indicator is a mixture of various PH indicators which give sharp colour at different PH.
Here HCL gives red colour and acetic acid gives yellow.
Activity 4.8
Brief procedure:
Activity 4.8 asks us to react glacial acetic acid with ethanol in the presence of acid and observe the smell.
Observation:
We observe a fruity smell from the solution.
Explanation:
Ethanol reacts with acetic acid in the presence of concentrated sulphuric acid and forms ester. Ester has a characteristic fruity or pleasant smell, which we can detect by the smell.
This is a reversible reaction, ethyl acetate produced is dilute after formation to avoid the backward reaction. Addition of bases like sodium hydroxide also reverses the reaction.
Activity 4.8
Brief procedure:
Activity 4.8 asks us to see if there is a reaction between ethanoic acid and carbonates or hydrogen carbonate like Activity 2.5.
Observation:
Similar to inorganic acids, ethanoic acid reacts with sodium carbonate and hydrogen carbonates. Evolved gas turns the lime water solution milky.
Explanation:
Carboxylic acids react with carbonates and hydrogen carbonate in the same fashion of inorganic acids and form respective salts. The only difference is the speed of the reaction. Here reaction takes place slowly.
Here sodium metal replaces the most polar hydrogen atom and forms the salt. Carbon dioxide gas evolves which turns the lime water milky.
Activity 4.10
Brief procedure:Activity 4.10 asks us to test the shake mixture of oil and water with soap and compare it with plain water and oil.
Observation:
The mixture of oil and water becomes cloudy with soap while oil separates and form a layer with plain water.
After some time the oil separates from both the solution. But it takes a longer duration for a cloudy solution.
Explanation:
Water is a polar compound. A polar substance like salt forms bond with polar hydrogen and hydroxide molecules of water. This polar nature makes salt soluble in water.
Oil is a non-polar compound. It does not form bonds with water molecules. As a result, oil does not dissolve in water. Instead, they start floating on the surface of the water as they are lighter than water.
Soaps are metal salts of long chain fatty acid. It’s one end is polar (Na+) while another end is non-polar. Polar end forms bonding with the water molecule and non-polar end forms bonding with oil molecules. This form the suspension of oil in water. As a result, the solution becomes cloudy. We call this process micelle formation
Application in daily life:
Dirt in our clothes is mainly oily substances. Soap or surf suspend the dirt between water and cloth. When we agitate this solution dirt passes out from the cloth, and we get a clean cloth.
Application in biological processes:
Pancreatic lipase is a water-soluble enzyme. It can not work on the separate layer of the long chain of the fatty acid of oils. Bile salt from liver suspends the fatty acid into the water to facilitate the action of lipase enzyme. We call this emulsification of fats. You will read this in detail in chapter 6 life processes.
Activity 4.11
Brief procedure:
Activity 4.11 asks us to check the amount of foam produced by soap in hard water and compare it with foam produced by soap in distilled water.
Observation:
We see more amount of foam in soap with distilled water.
Explanation:
Hard water contains some amount of hydrogencarbonates, sulphates, and chlorides of calcium and magnesium. These salts displace sodium from the soap and form fatty acid of magnesium and calcium. These salts are insoluble and deposit on the bottom as scum. As a result, less soap is available to form the foam
Hard water reaction with soap.
Activity 4.12
Brief procedure:
Activity 4.12 asks us to compare the foam produced by a soap with hard water and by a detergent with hard water.
Observation:
There is more foam production with detergent.
Explanation:
Soaps are sodium salts of naturally occurring fatty acids like palmitic acid, stearic acid etc. We prepare them by the saponification of natural oils like coconut oil, butter, olive oil with salt. They react with calcium and magnesium ions present in hard water and form an insoluble precipitate. This process reduces the quantity of soap available. Thus soap forms less foam.
Hard water reaction with soap.
Detergents are chemically synthesised compounds also known as cleansing agents. They generally consist of the sodium salt of sulphonic acid or ammonium salt with bromine and chloride ions. These do not react with ions present in hard water. As a result, there is a lesser influence of hard water on foam formation. As a result, we see more foam production with detergents than soaps.











No comments:
Post a Comment