Class 10 Science
Chapter 1 Chemical Reactions and Equations
Activities Solutions
Activity 1.1
Brief Procedure: Activity 1.1 asks us to burn Magnesium ribbon in a china dish and see what happens.
Observation: Magnesium ribbon burns spontaneously, and white ash deposits on the china dish.
Magnesium is a highly reactive metal. It reacts spontaneously with oxygen present in the atmosphere to form white ash of Magnesium oxide with the liberation of energy.
2Mg(s) + O2(g) ———> 2MgO(s)
Caution:
Magnesium can burn spontaneously like a cracker. Keep it away from the body during the experiment.
Activity 1.2
Brief procedure: Activity 1.2 asks us to mix an aqueous solution of lead nitrate with potassium iodide to check what happens.
Observation: A yellow colour precipitate appears at the bottom.
Explanation: Lead nitrate and potassium iodide; both are colourless. They react with each other to form a yellow precipitate of lead iodide. Lead iodide settles down at the bottom of the tube.
Pb(NO3)2(aq)+ 2KI(aq) → PbI2(s) + 2KNO3(aq)
Activity 1.3
Objective: The question ask to put zinc granules in the beaker containing acid either hydrochloric acid or sulphuric acid and ask for what we observe.
Observation: Air Bubbles comes out from the granules, and Conical flask becomes warm.
Inference: Zinc granules react with hydrochloric acid or sulfuric acid and forms hydrogen gas.
Zn(s) + 2HCl(aq) → ZnCl2(aq)+ H2↑ + heat
Zn(s) + 2H2SO4(aq) → ZnSO4(aq) + 2H2↑ + heat
Caution: Acids are corrosive and harmful for skin. Avoid touching them with bare skin.
Activity 1.4
Brief Procedure: This activity asks to put some quick lime (CaO) into the water and observe the reaction.
Observation: Beaker feel hot after adding water
Explanation: Quick lime reacts with water to form slaked lime. The process is exothermic and releases heat.
CaO(s) + H20(l) → Ca(OH)2(aq) + Heat
Application in whitewashing: Slaked lime reacts with the carbon dioxide present in the air. It forms Calcium carbonate which is a shiny compound. For example, Marble used in the home is also the same. So we use slaked lime as whitewash paint in home walls. After two to three days slaked lime convert to carbonate which gives the wall a shiny surface.
Ca(OH)2(aq) + CO2(g) ——–> CaCO3(s) + H20(l)
Another fact: We all know human, and animals absorb oxygen from air and emit carbon dioxide. When we blow air from the mouth into slaked lime solution using a tube, an insoluble precipitate of calcium carbonate forms. This phenomenon confirms the presence of C02 in our breath.
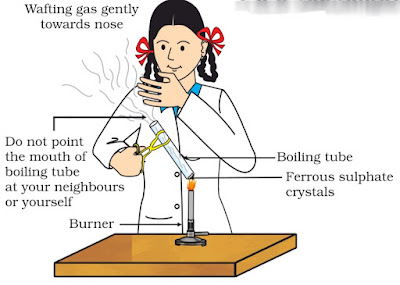
Activity 1.5
: Activity 1.5 asks us to heat ferrous sulphate crystals (aka Green vitriol) in a test tube and see what happens.
Observation: The Green colour of ferrous sulphate changes to brown and peculiar sulphur smell comes out from the test tube.

Inference: Heating ferrous sulphate on test tube leads to decomposition of ferrous sulphate into a ferric oxide which is brown. It also liberates sulphur dioxide gas which has a foul smell.
2FeSO4(S) ———-> Fe2O3(s) + SO2(g) + SO3(g)
Activity 1.6
Procedure: The question asks to heat an aqueous solution of lead nitrate into a test tube and see what happens.
Observation: A yellow precipitate of lead oxide form with the evolution of brown nitrogen dioxide gas which has an irritating smell.
Inference: Lead nitrate decomposes to lead oxide which is yellow. Nitrogen dioxide gas is liberated which has a brown colour and irritating smell.
2Pb(NO3)2(s) ———> 2PbO(s) + 4NO2(g) + O2(g)
Lead nitrate Heat Lead oxide Nitrogen oxide Oxygen
Activity 1.7
Procedure: The activity asks to electrolyse water using a 6-volt battery and check the volume of gases liberated at anode and cathode. The activity also asks to bring the gas at the flame and see what happens.
Observation:
1. Gas collected at the cathode (-) is twice that of gas collected at the anode (+).
2. When we bring the flame to the gas collected at the cathode, it burns immediately. While gas obtained at anode does not burn.
Inference: Electricity decomposes water into its component. Hydrogen atoms in water receive an electron from the cathode electrode and form hydrogen gas. Similarly, oxygen atoms in water lose electrons at the anode and form oxygen gas.
Explanation: We can explain this with the equation below.
2H2O ——> 2H2 + O2
Here you can see two molecules of hydrogen gas liberate with one molecule of oxygen. So the volume of hydrogen obtained is double the amount of oxygen.
Note: water is not highly conductive. We use a few drops of sulphuric acid here. It is done to increase the electrical conductivity of water. Instead of sulphuric acid, any other acid like hydrochloric acid or salt like sodium chloride can also work.
Procedure: The concerned activity asks us to put silver chloride in sunlight for a few minutes.
Observation: Colour of silver chloride turns grey from white.
Explanation: Silver chloride in presence of light decomposes to solid silver and chlorine gas. Elemental silver is grey in colour, so a grey colour appears.
( Sunlight)
2AgCl(s) ———> 2Ag(s) + Cl2(g)
Application: Photographic film
Silver chloride (and all halides of silver) are photosensitive. They change the colour on exposure to light. This property is used in the photographic industry to make films. Films have a thin layer of silver chloride coated on them. When we click the camera, light passes through the lens to the film and image is imprinted on the film. Processing this film results in coloured photos which we keep in albums and posters.
Note: We prepare Silver chloride by mixing silver nitrate solution with sodium chloride solution. A milky white precipitate is the silver chloride.
AgNO3 + NaCl ———> AgCl↓ + NaNO3
Activity 1.9
Brief Procedure:
Activity 1.9 asks us to dip iron nails in a copper sulphate solution and check the colour of the solution.
Observation: Colour of copper solution fades and nail becomes brown.Note: The faded blue apperance later change to green colour because ferrous sulphate is green.
Activity 1.10
Brief Procedure: Activity 1.10 asks us to mix the sodium sulphate solution with barium chloride solution.
Observation: A white precipitate of barium sulphate sinks at the bottom.
Explanation: Sodium sulphate and barium chloride undergo double displacement and form barium sulphate and sodium chloride. The reaction goes in the forward direction as barium sulphate formed is insoluble in water and sinks to the bottom.
Na2SO4 (aq)+ BaCl2(aq) —> BaSO4(white ppt.) + 2NaCl(aq)
Inference: Sodium sulphate and barium chloride undergo double-displacement reaction and form insoluble white barium sulphate precipitate.
Activity 1.11
Procedure: This activity asks us to heat copper powder in a china dish.
Observation: Surface of powder becomes black.
Explanation: All metals except gold and platinum are reactive elements. They react with various gases present in the atmosphere like oxygen, sulphur etc. These reactions take time. Heating metals hastens the reaction and we can easily observe the change during heating. Copper also reacts with atmospheric oxygen and forms a coating of its oxide on them. This oxide gives them a black appearance.
Heat
2Cu(s) + O2(g) ————> CuO(s)
Some extra facts: Copper is used in many forms as wires; as kitchen utensils; in an idol etc. Oxidation of copper with times makes them black. Most of the time these products also have a green appearance. This phenomenon occurs due to the reaction of oxide with sulphur present in the atmosphere.
Acidic substances like vinegar, lemon remove this layer. So we use them to clean our copper products.



















No comments:
Post a Comment