Class 10 Science
Chapter 4 Carbon and its Compounds
Topics in the Chapter
• Introduction
• The Covalent Bond
• Versatile nature of Carbon
• Chemical Properties of Carbon Compounds
• Important Carbon Compounds
• Soaps and Detergents
Introduction
Occurrence of carbon :-
i) Carbon is found in the atmosphere, inside the earth’s crust and in all living organisms.
ii) Carbon is present in fuels like wood, coal, charcoal, coke, petroleum, natural gas, biogas, marsh gas etc.
iii) Carbon is present in compounds like carbonates, hydrogen carbonates etc.
iv) Carbon is found in the free state as diamond, graphite, fullerenes etc.
The Covalent Bond
→ Carbon always forms covalent bond.
→ The bond formed by sharing of electron pair between two atoms are known as covalent atoms.
• Noble gas configuration of Carbon
→ Carbon is tetravalent, it does not form ionic bond by either losing four electrons (C4+) or by gaining four electrons (C4-). It is difficult to hold four extra electron and would require large amount of energy to remove four electrons. So, carbon can form bond by sharing of its electrons with the electrons of other carbon atom or with other element and attain noble gas configuration.
→ The atoms of other elements like hydrogen, oxygen and nitrogen, chlorine also form bonds by sharing of electrons.
Formation of covalent bonds :-
Covalent bond is chemical bond formed by the sharing of electrons between atoms.
The sharing of one pair of electrons results in the formation of single covalent bond, sharing of two pairs of electrons results in the formation of double covalent bond and sharing of three pairs of electrons results in the formation of triple covalent bond.
• H – H single bond between hydrogen atoms (H2)
The atomic number of hydrogen is 1, its electronic arrangement is 1, it has 1 valence electron. It needs 1 electron more to attain stability. So two hydrogen atoms share 1 pair of electrons resulting in the formation of a single covalent bond in hydrogen molecule H2.



→ Covalent compounds have low melting and boiling points as they have weak intermolecular force.

→ They are generally poor conductor of electricity as electrons are shared between atoms and no charged particles are formed.
Versatile Nature of Carbon
The two characteristic properties of carbon element which lead to the formation of large number of compounds
• Catenation: Carbon can link with carbon atoms by means of covalent bonds to form long chains, branched chains and closed ring Compounds.
Carbon atoms may be linked by single, double or triple bonds.
• Tetravalency: Carbon has 4 valence electrons. Carbon can bond with four carbon atoms, monovalent atoms, oxygen, nitrogen and sulphur.
Hydrocarbons, Saturated and Unsaturated hydrocarbons :-
i) Hydrocarbons :- are compounds containing carbon and hydrogen
atoms.
ii) Saturated hydrocarbons :- are hydrocarbons having all single
covalent bonds between the carbon atoms.
Eg : Alkanes :- have all single covalent bonds between the carbon
atoms and their names end with – ane.
Methane CH4, Ethane C2H6
iii) Unsaturated hydrocarbons :- are hydrocarbons having a double or
triple covalent bond between two carbon atoms. Eg : Alkenes and
Alkynes.
Alkenes :- have a double covalent bond between two carbon atoms.
and their names end with – ene. Ethene C2H4, Propene C3H6
Alkynes :- have a triple covalent bond between two carbon atoms
and their names end with – yne.
Ethyne C2H2, Propyne C3H4
Electron Dot Structure of Saturated Hydrocarbons
Methane CH4
Ethane C2H6
Names, molecular formulae and structure formulae of saturated hydrocarbons (Alkanes):
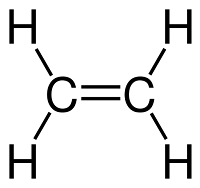
Ethene (C2H4)
Ethyne (C2H2)
Names, molecular formulae and structure formulae of unsaturated hydrocarbons (Alkenes and Alkynes):

Isomerism
Carbon compounds having the same molecular formula but different structural formulae are called isomers. This property is called isomerism.
Eg:- Butane – C4H10 has 2 isomers. They are Normal butane and Iso butane.
Pentane – C5H12 has 3 isomers. They are Normal pentane, Iso pentane and Neo
pentane.
Functional groups
An atom or a group of atoms which decides the properties of a carbon compound is called a functional group.
i) Halide ( Halo group) :- - Cl, - Br, etc. ( Names end with – ane)
Eg :- CH3Cl – Chloro methane, C2H5Br – Bromo ethane
ii) Alcohol :- - OH ( Names end with – ol)
Eg :- CH3OH – Methanol, C2H5OH – Ethanol
iii) Aldehyde :- - CHO ( Names end with – al )
Eg :- HCHO – Methanal, CH3CHO – Ethanal
iv) Carboxylic acid :- - COOH (Names end with – oic acid)
Eg :- HCOOH – Methanoic acid, CH3COOH – Ethanoic acid
v) Ketone :- (Names end with – one )
Eg :- CH3COCH3 – Propanone , CH3COC2H5 - Butanone
Homologus series
Homologus series is a group of carbon compounds having similar
structures, similar chemical properties and whose successive members
differ by a – CH2 group. Eg :- Alkanes, Alkenes, Alkynes etc.
Alkanes :- have general molecular formula CnH2n+2 . Their names end
with – ane and the members are as follows :-
Methane - CH4
Ethane - C2H6
Propane - C3H8
Butane - C4H10
Pentane - C5H12
Alkenes :- Alkenes have general molecular formula CnH2n . Their names end with – ene and the members are as follows :-
Ethene - C2H4
Propene - C3H6
Butene - C4H8
Pentene - C5H10
Alkynes :- Alkynes have general molecular formula CnH 2n – 2 .Their names end with – yne and the members are as follows :-
Ethyne - C2H2
Propyne - C3H4
Butyne - C4H6
a) Combustion :-
Carbon compounds burn in oxygen to form water, carbon dioxide,
heat and light.
Eg :- C + O2 --> CO2 + heat + light
CH4 + 2O2 --> 2H2O + CO2 + heat + light
C2H5OH + 3O2 --> 3H2O + 2CO2 heat + light
b) Oxidation :-
Carbon compounds like alcohols are oxidised to carboxylic acids on
heating with oxidising agents like alkaline Potassium permanganate
– KMnO4 or acidic potassium dichromate - K2Cr2O7 .
Eg:- Alcohols are oxidised to Carboxylic acids
alkaline KMnO4 + heat
C2H5OH --> CH3COOH
Ethanol acidic K2Cr2O7 + heat Ethanoic acid
c) Addition reaction :-
Unsaturated hydrocarbons undergo addition reaction with hydrogen in the presence of nickel or palladium as catalyst to form saturated hydrocarbons.
Eg:- Ethene undergoes addition reaction with hydrogen to form ethane in the
presence of nickel or palladium as catalyst.
Ni or Pd catalyst
C2H4 + H2 --> C2H6
H H H H
I I Ni or Pd catalyst I I
C = C + H2 --> H – C – C – H
I I I I
H H H H
The addition of hydrogen to unsaturated hydrocarbons to form saturated hydrocarbons is called hydrogenation. Hydrogenation is used to convert unsaturated oils and fats to saturated oils and fats.
d) Substitution reaction :-
Saturated hydrocarbons undergo substitution reaction with halogens to form substitution products.
Eg :- Methane undergoes substitution reaction with chlorine in the presence of sunlight to form substitution products.
CH4 + Cl2 --> CH3Cl + HCl CH3Cl + Cl2 --> CH2Cl2 + HCl
CH2Cl2 + Cl2 --> CHCI3 + HCl CHCI3 + Cl2 --> CCl4 + HCl
Some important carbon compounds
a) ETHANOL :- C2H5OH - Ethyl alcohol
Properties :-
i) Ethanol is a colourless liquid with a pleasant smell and burning
taste.
ii) It is soluble in water.
iii) Ethanol reacts with sodium to form sodium ethoxide and hydrogen.
2C2H5OH + 2Na --> 2C2H5ONa + H2
iv) Ethanol reacts with hot conc. H2SO4 to form ethene and water. Conc.
H2SO4 is a dehydrating agent and removes water from ethanol.
conc. H2SO4
C2H5OH --> C2H4 + H2O
Uses :-
i) Ethanol is used for making alcoholic drinks.
ii) It is used as a solvent.
iii) It is used for making medicines like tincture iodine, cough syrups,
tonics etc.
b) ETHANOIC ACID :- CH3COOH – Acetic acid
Properties :-
i) Ethanoic acid is a colourless liquid with a pungent smell and sour taste.
ii) It is soluble in water.
iii) A solution of 5% to 8% ethanoic acid in water is called Vinegar.
iv) Esterification :-
Ethanoic acid reacts with ethanol to form the ester ethyl ethanoate in the presence of conc. H2SO4.
conc.H2SO4
CH3COOH + C2H5OH --> CH3COOC2H5 + H2O
The reaction between carboxylic acid and alcohol to form an ester is called
esterification.
v) Saponification :-
When an ester reacts with sodium hydroxide solution, the sodium salt of the
carboxylic acid and the parent alcohol are formed. This reaction is called
saponification.
Eg :-Ethyl ethanoate reacts with sodium hydroxide to form sodium acetate and ethanol.
CH3COOC2H5 + NaOH --> CH3COONa + C2H5OH
vi) Ethanoic acid reacts with bases to form salt and water.
CH3COOH + NaOH --> CH3COONa + H2O
vii) Ethanoic acid reacts with carbonates and hydrogen carbonates to form salt, water
and carbon dioxide.
2CH3COOH + Na2CO3 --> 2CH3COONa + H2O + CO2
CH3COOH + NaHCO3 --> CH3COONa + H2O + CO2
Soaps and detergents
a) Soaps :- Soaps are long chain sodium or potassium salts of carboxylic acids. Eg:- Sodium stearate – C17H35COONa
Structure of soap molecule :- A soap molecule has two parts. A long hydrocarbon part which is hydrophobic (water repelling) and soluble in oil and grease and a short ionic part which is hydrophyllic (water attracting) and insoluble in oil and grease.
Cleansing action of soap :- When soap is dissolved in water it forms spherical structures called micelles. In each micelle the soap molecules are arranged radially such that the HC part is towards the centre and the ionic part is towards the outside. The HC part dissolves the dirt, oil and grease and forms an emulsion at the centre of the micelles which can be washed away by water.
Detergents :-
Detergents are long chain sodium salts of sulphonic acids. Soaps do not wash well with hard water because it forms insoluble precipitates of calcium and magnesium salts in hard water. Detergents wash well with hard water because it does not form insoluble precipitates of calcium and magnesium salts in hard water.
Differences between soaps and detergents :-




.png)
.png)



















No comments:
Post a Comment